Inventory Management Built for Pharmaceutical Manufacturers
Meet FDA GMP requirements with comprehensive batch records, complete lot traceability, component release workflows, and electronic documentation—all designed specifically for pharmaceutical manufacturing compliance.
Challenges Pharmaceutical Manufacturers Face
The pharmaceutical industry faces intense FDA scrutiny. Managing GMP compliance, documentation, and traceability with inadequate tools puts your license and patients at risk.
FDA GMP Compliance Pressure
Meeting 21 CFR Parts 210 and 211 requirements for batch production records, laboratory records, and component documentation is complex and high-stakes.
Incomplete Batch Documentation
Paper-based or spreadsheet batch records miss critical details, lack proper sign-offs, and create compliance gaps that FDA inspectors will find during audits.
Lot Traceability Gaps
When an API or excipient supplier has a quality issue, tracing affected drug products lot-by-lot must happen within hours for patient safety.
Component Release Bottlenecks
Managing quarantine, sampling, testing, CoA review, and release decisions for incoming components without proper systems delays production.
Expiration & Stability Management
Without proper systems, products ship close to expiration or components are used past their retest dates. Stability data lives in disconnected files.
21 CFR Part 11 Requirements
Electronic records require audit trails, electronic signatures, and access controls. Generic spreadsheets do not meet these requirements.
How Fiddle Helps Pharmaceutical Manufacturers Stay Compliant
Built specifically for pharmaceutical manufacturers, Fiddle provides the GMP documentation, traceability, and quality tools required by FDA regulations.
GMP-Compliant Batch Records
Create master batch records and batch production records that meet 21 CFR Part 211 requirements. Include all required documentation with digital sign-offs.
Learn more →Complete Lot Traceability
Track every API, excipient, and packaging component from receipt through distribution. Run forward and backward traces in seconds for rapid recall response.
Learn more →Component Release Workflow
Manage quarantine, sampling, testing, CoA review, and release decisions. Prevent unreleased materials from entering production automatically.
Expiration & Retest Date Tracking
Track component and finished product expiration dates. Manage retest dates and stability data. Enforce FIFO and prevent expired material use.
Formula & Potency Management
Build product formulas with exact potencies and specifications. Track label claims against actual formulation to ensure products meet specifications.
Learn more →Production Planning & Control
Visual work order management with automatic component allocation. Schedule production based on component availability and release status.
Learn more →Features Built for FDA-Regulated Pharmaceutical Manufacturing
Everything you need to maintain GMP compliance, manage production, and ensure patient safety.
FDA GMP Compliant Batch Records
Create master batch records (MBRs) and batch production records (BPRs) that satisfy FDA 21 CFR Part 211 requirements. Document component dispensing, processing steps, in-process testing, equipment cleaning, and operator sign-offs—all with complete audit trails.
- Master batch record templates with all required fields
- Batch production records with unique lot numbers
- Component identity, strength, quality, and purity verification
- In-process and release testing documentation
- Digital signatures with 21 CFR Part 11 considerations
- Deviation, investigation, and CAPA documentation
- Equipment cleaning and line clearance records
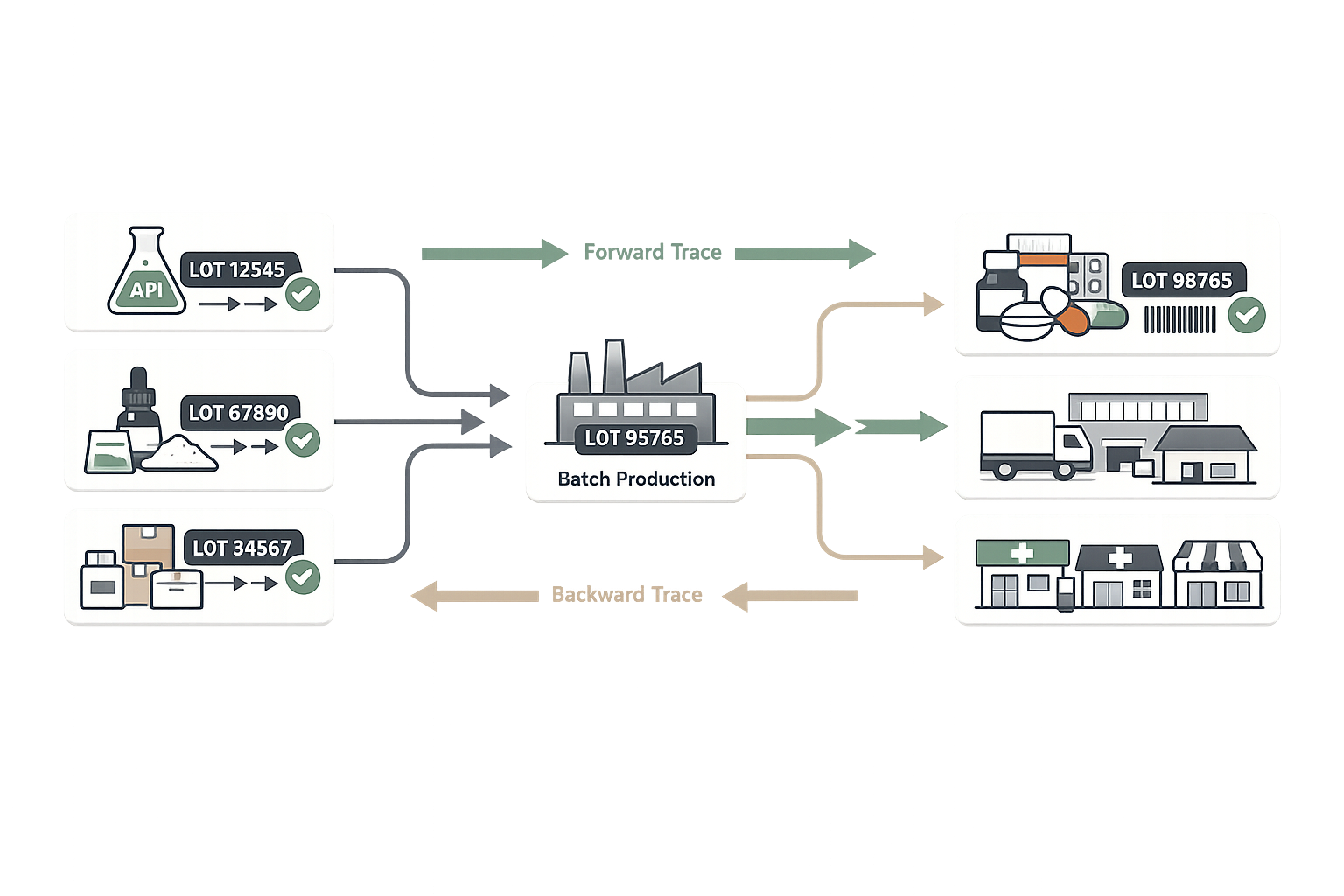
Component-to-Distribution Lot Traceability
Achieve complete forward and backward traceability from raw APIs and excipients through finished drug product distribution. Meet FDA requirements for rapid recall capability and respond to quality issues within hours, not days.
- Automatic lot number generation with customizable formats
- Supplier lot capture and certificate of analysis linkage
- Forward trace: find all products containing a component lot
- Backward trace: identify all components in a finished product lot
- Distribution lot tracking for recall execution
- One-click trace reports for FDA inspections
- Complete chain of custody documentation
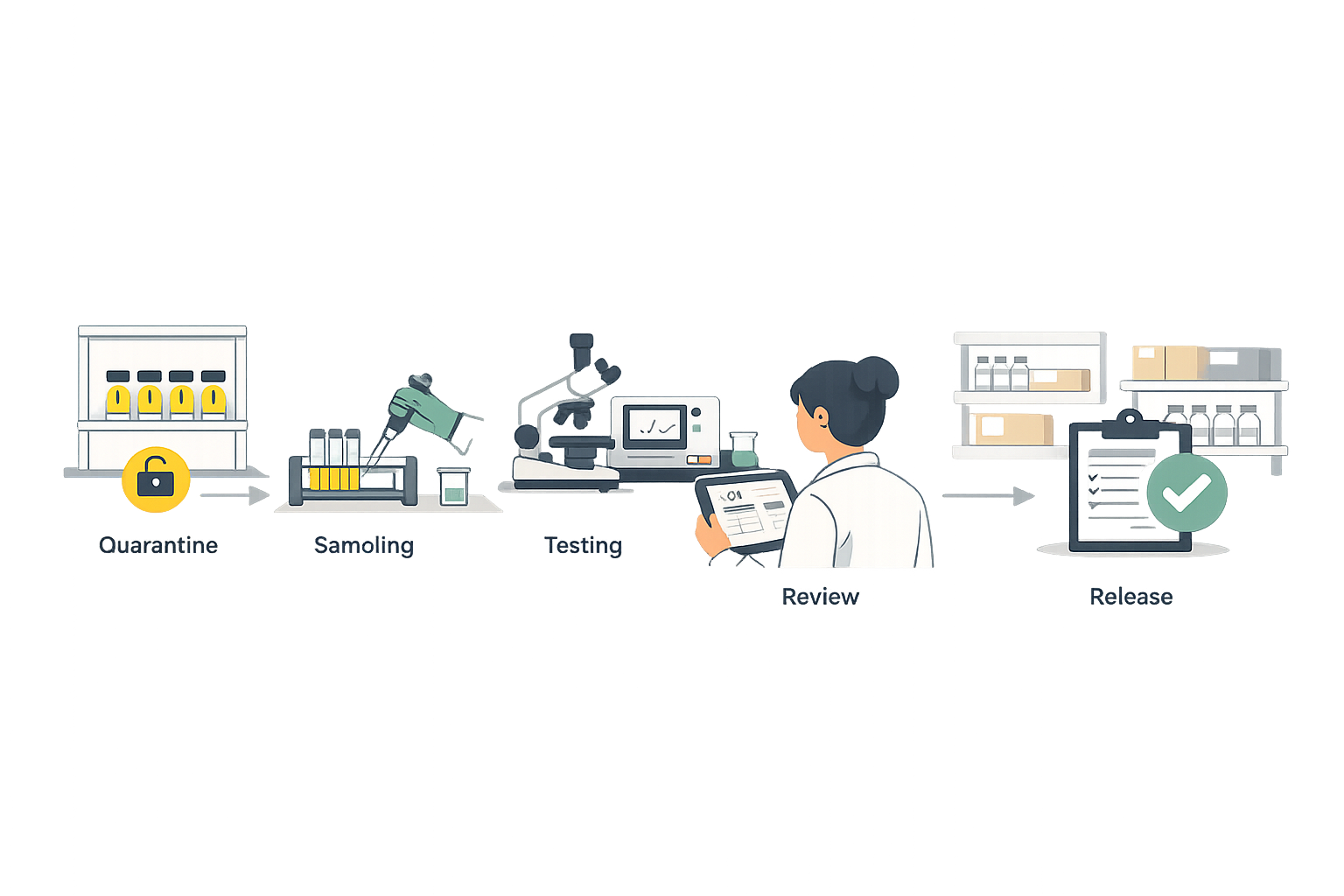
Component Quarantine & Release Management
Manage the complete component qualification process from receipt through release. Handle quarantine, sampling, identity testing, CoA review, and release decisions with proper approval workflows. Prevent unreleased components from entering production.
- Automatic quarantine status at receiving
- Sampling and testing assignment workflows
- Certificate of Analysis attachment and review
- Identity testing documentation and approval
- Hold, release, and reject status management
- Specification conformance verification
- Approved supplier and component qualification
Explore Key Features for Pharmaceutical
Discover how Fiddle's purpose-built features help pharmaceutical manufacturers streamline operations, maintain compliance, and grow their business.
21 CFR Part 211 Batch Records
Create GMP-compliant batch production records with all required documentation.
Learn More →Component Lot Traceability
Track APIs, excipients, and packaging from receipt through distribution.
Learn More →Formula & Potency Management
Build drug product formulas with exact potencies and specifications.
Learn More →Production Planning
Schedule production based on component availability and release status.
Learn More →Solutions for Pharmaceutical
Explore how Fiddle's specialized solutions help pharmaceutical businesses solve their unique challenges and achieve operational excellence.
FDA cGMP Compliance
Built-in compliance controls for 21 CFR Part 211 GMP requirements.
View Compliance Features →Batch Record Management
Create GMP-compliant batch production records with all required documentation.
Explore Batch Records →Lot Traceability
Track APIs, excipients, and packaging from receipt through distribution.
Learn about Lot Traceability →Bill of Materials
Build drug product formulas with exact potencies and specifications.
View Formula Management →Production Scheduling
Schedule production based on component availability and release status.
Discover Production Scheduling →Expiration Tracking
Track expiration dates and stability data for APIs and finished products.
See Expiration Tracking →Trusted by Pharmaceutical Manufacturers
See why pharmaceutical companies choose Fiddle for their GMP compliance, inventory, and production needs.
Our FDA inspection went smoother than any we have had in 15 years. The inspector spent an entire day reviewing batch records and traceability. Her comment was that our documentation was exceptionally well-organized. That is Fiddle.
When we had a supplier recall on an API lot, we traced all affected finished products and initiated our recall within 4 hours. Before Fiddle, that would have been a multi-day, all-hands effort.
The component release workflow eliminated our quarantine backlog. Materials used to sit for weeks waiting for paperwork. Now our QA team processes releases the same day because everything is in one system.
Trusted by leading brands




Ready to Simplify GMP Compliance for Your Pharmaceutical Operations?
Join pharmaceutical manufacturers who use Fiddle to maintain FDA compliance, manage production, and ensure patient safety. Get started with a free demo today.