Inventory Management Built for Supplement Manufacturers
Meet FDA 21 CFR Part 111 requirements with comprehensive batch records, complete lot traceability, CoA management, and potency tracking—all designed specifically for dietary supplement and nutraceutical manufacturers.
Challenges Supplement Manufacturers Face Every Day
The dietary supplement industry faces intense FDA scrutiny. Managing cGMP compliance, documentation, and traceability with inadequate tools puts your business at risk.
FDA cGMP Compliance Pressure
Meeting 21 CFR Part 111 requirements for batch production records, master manufacturing records, and component documentation is complex and high-stakes.
Incomplete Batch Documentation
Paper-based or spreadsheet batch records miss critical details, lack proper sign-offs, and create compliance gaps that auditors will find.
Traceability Gaps Cost Time and Money
When an ingredient supplier has a potency or contamination issue, tracing affected finished products lot-by-lot takes days instead of minutes.
CoA and Specification Management Chaos
Managing certificates of analysis, identity testing results, and component specifications across dozens of ingredients without a system is error-prone.
Potency and Stability Tracking Failures
Without proper systems, products ship with expired or out-of-spec ingredients. Stability testing data lives in disconnected files.
No Real-Time Production Visibility
Disconnected systems mean you cannot see true inventory levels, production status, or what can actually be manufactured today.
How Fiddle Helps Supplement Manufacturers Stay Compliant
Built specifically for dietary supplement manufacturers, Fiddle provides the cGMP documentation, traceability, and production tools required by FDA regulations.
cGMP-Compliant Batch Records
Create master manufacturing records and batch production records that meet 21 CFR Part 111 requirements. Include all required documentation automatically.
Learn more →Complete Component Traceability
Track every dietary ingredient and component from receipt through finished product distribution. Run forward and backward traces in seconds.
Learn more →CoA & Specification Management
Attach certificates of analysis, identity test results, and specifications to component lots. Ensure only verified materials release for production.
Expiration & Potency Tracking
Track component and finished product expiration dates. Manage retest dates and stability data. Enforce FIFO and prevent expired material use.
Formula & Label Claim Management
Build product formulas with exact potencies and overages. Track label claims against actual formulation to ensure product meets specifications.
Learn more →Production Planning & Allocation
Visual work order management with automatic component allocation. See exactly what you can produce based on available, released inventory.
Learn more →Features Built for FDA-Regulated Supplement Manufacturing
Everything you need to maintain cGMP compliance, manage production, and grow your supplement business.
21 CFR Part 111 Compliant Batch Records
Create master manufacturing records (MMRs) and batch production records (BPRs) that satisfy FDA cGMP requirements. Document component identity verification, in-process testing, equipment calibration, and operator sign-offs—all with complete audit trails.
- Master manufacturing record templates with required fields
- Batch production records with unique batch numbers
- Component identity verification documentation
- In-process and finished product testing records
- Digital signatures with 21 CFR Part 11 considerations
- Deviation and CAPA documentation within batch records
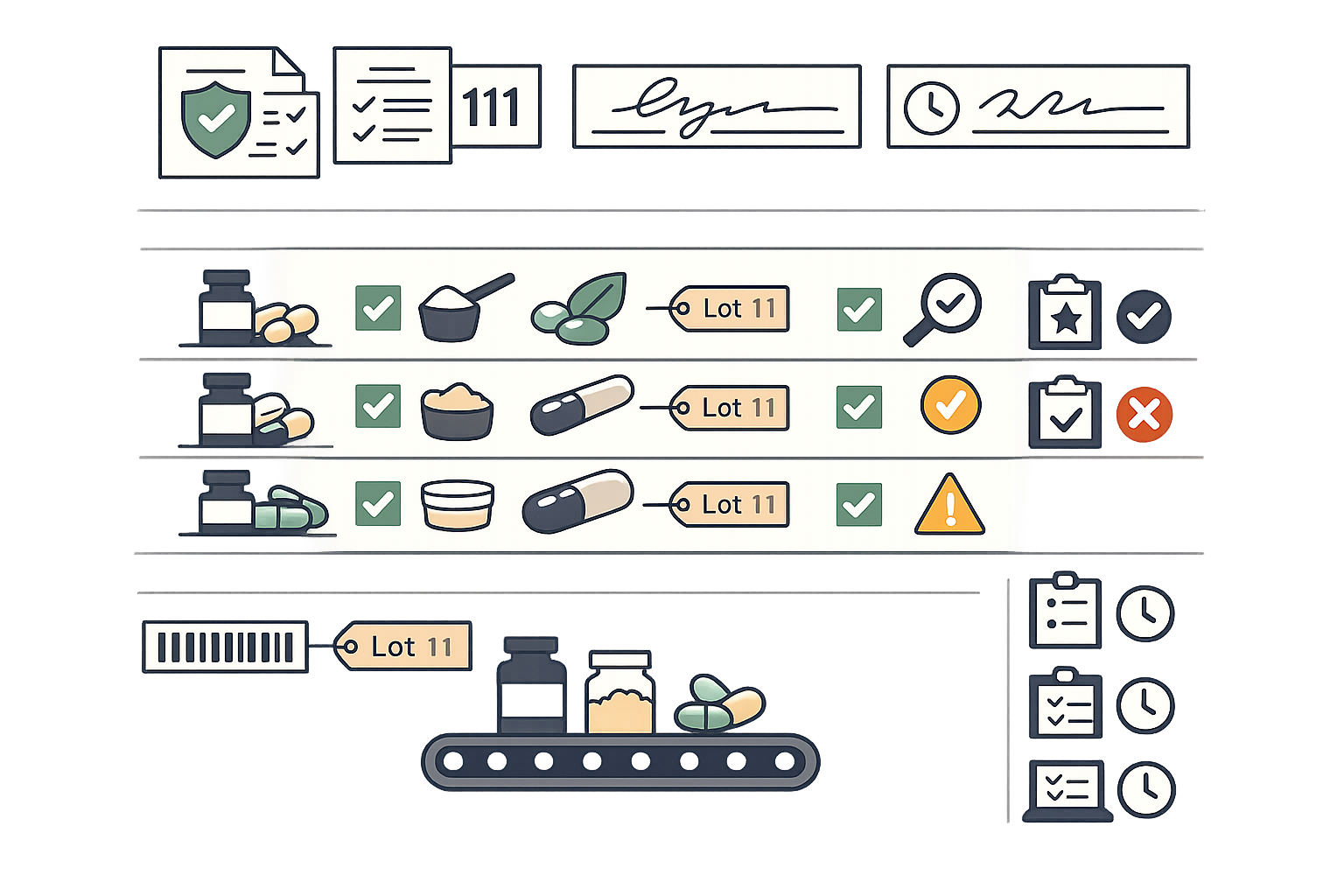
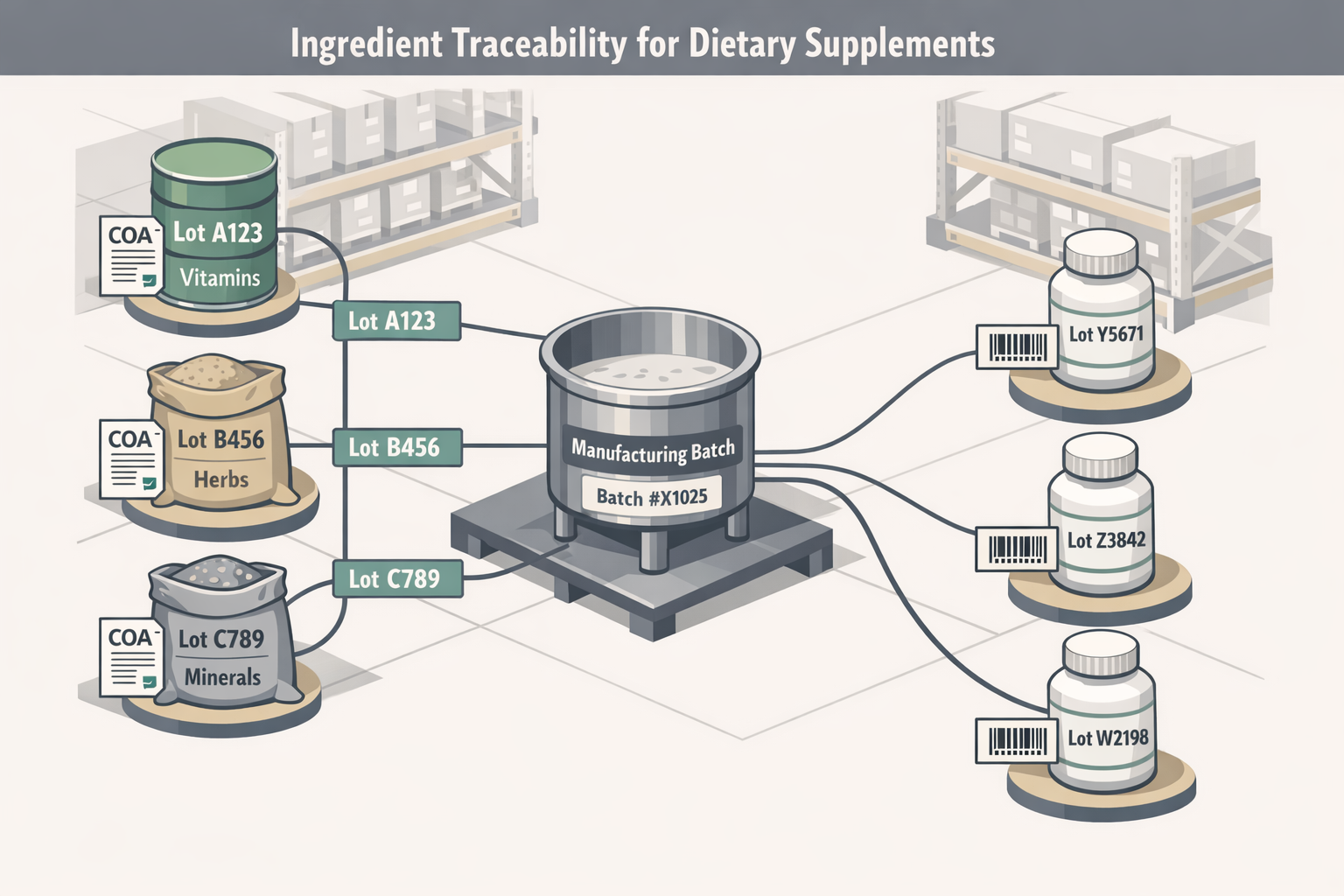
Component-to-Distribution Traceability
Achieve complete forward and backward traceability from raw dietary ingredients through finished product distribution. Meet FDA requirements for one-step-up, one-step-back traceability and respond to recalls within hours, not days.
- Automatic lot number generation with customizable formats
- Supplier lot capture and certificate of analysis linkage
- Forward trace: find all products containing a specific component lot
- Backward trace: identify all components in a finished product lot
- Distribution lot tracking for recall execution
- One-click trace reports for FDA inspections
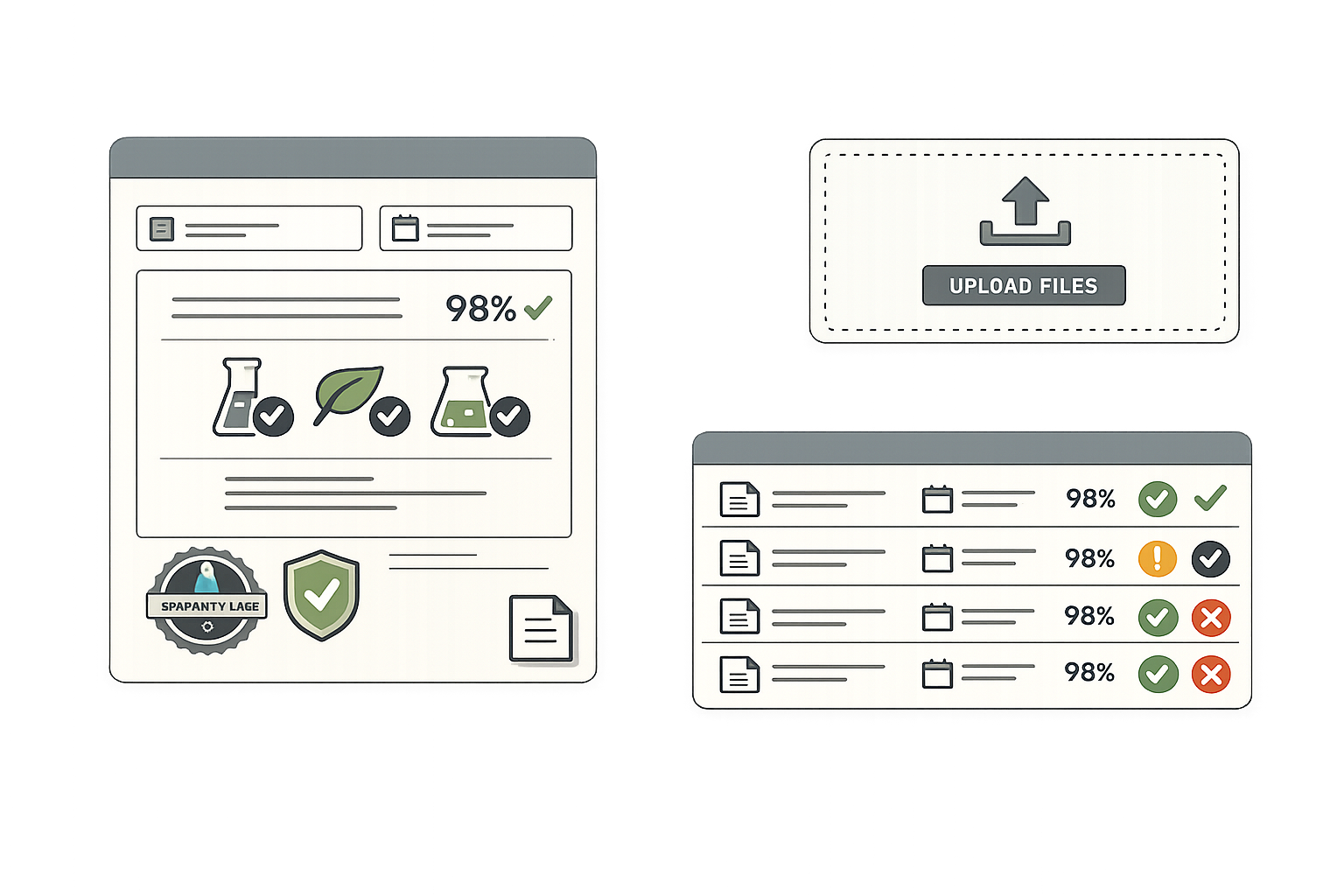
Component Release & CoA Management
Manage the entire component qualification process from receipt through release. Attach certificates of analysis, identity testing results, and specifications to each lot. Prevent unreleased components from entering production.
- Receiving inspection and quarantine status tracking
- Certificate of Analysis attachment and review workflow
- Identity testing documentation and sign-off
- Specification conformance verification
- Hold, release, and reject status management
- Allergen and claim documentation at lot level
Explore Key Features for Supplements & Nutraceuticals
Discover how Fiddle's purpose-built features help supplements & nutraceuticals manufacturers streamline operations, maintain compliance, and grow their business.
21 CFR Part 111 Batch Records
Create cGMP-compliant master manufacturing records and batch production records.
Learn More →Component Lot Traceability
Track dietary ingredients from receipt through distribution for FDA compliance.
Learn More →Formula & Potency Management
Build product formulas with exact potencies and manage label claim compliance.
Learn More →Solutions for Supplements & Nutraceuticals
Explore how Fiddle's specialized solutions help supplements & nutraceuticals businesses solve their unique challenges and achieve operational excellence.
FDA cGMP Compliance
Built-in compliance controls for 21 CFR Part 111 cGMP requirements and FDA regulations.
View Compliance Features →Batch Record Management
Create master manufacturing records and batch production records with digital signatures and audit trails.
Explore Batch Records →Lot Traceability
Track dietary ingredients from receipt through distribution for complete FDA compliance.
Learn about Lot Traceability →Bill of Materials
Build product formulas with exact potencies and manage label claim compliance.
Explore BOM Management →Expiration Tracking
Automated expiration date management with FIFO enforcement for ingredient freshness.
See Expiration Tracking →Production Scheduling
Visual production planning with automatic component allocation and release status tracking.
Discover Production Scheduling →Trusted by Dietary Supplement Manufacturers
See why leading supplement brands choose Fiddle for their cGMP compliance, inventory, and production needs.
When FDA came for our inspection, they spent an hour going through our batch records and traceability documentation. The inspector commented that our records were some of the most complete she had seen from a company our size. That is Fiddle.
We used to spend 20+ hours per week managing spreadsheets, CoAs, and batch documentation. Fiddle automated most of that. Now we spend that time on product development and growth instead of compliance paperwork.
The component traceability feature saved us during a supplier recall last year. We identified all affected products and lots within an hour. Without Fiddle, that would have taken days and we might have missed something.
Trusted by leading brands




Ready to Simplify cGMP Compliance for Your Supplement Business?
Join leading dietary supplement manufacturers who use Fiddle to maintain FDA compliance, manage production, and grow their business. Get started with a free demo today.